Dr. Robert H. Zeiler, Professor of Pathophysiology, School of Pharmacy and Professor of Physiology, School of Medicine.
BIO
Dr. Robert H. Zeiler, Professor of Pathophysiology, School of Pharmacy and Professor of Physiology, School of Medicine, has scientific degrees from Long Island University (B.S., Biology, 1971 and M.S. Physiology, 1973) and earned his doctorate in Biology from New York University, Washington Square, 1981. Prior to entering the pharmaceutical industry, he performed original research in cardiac electrophysiology and biophysics, particularly in arrhythmia and ischemia, publishing in over 50 abstracts, presentations and peer-reviewed journals.
EDUCATION
1981
Doctor of Philosophy, Biology
New York University
Graduate School of Arts and Science
Washington Square
New York, New York, USA
1973
Master of Science, Physiology
Long Island University
Flatbush Avenue Extension
Brooklyn, New York, USA
1971
Bachelor of Science, Biology
Long Island University
Flatbush Avenue Extension
Brooklyn, New York, USA
MEMBERSHIPS
1995 to Present American Heart Association
2010 to Present Society of Quality Assurance
PUBLICATIONS AND PRESENTATIONS
1. Gelles JM and Zeiler RH: “ELECTRO-MECHANICAL COUPLING IN CARDIAC MUSCLE; EFFECTS OF CALCIUM IONOPHORES.” Circulation, 55 and 66: III-46, 1977.
2. Gelles JM and Zeiler RH: “IS NA-CA EXCHANGE IN CARDIAC PURKINJE FIBERS ELECTROGENIC?.” Fed. Proceedings, 37:3, 1987, 1978.
3. Zeiler RH, Gelles JM, and Krasnow N: “THE EFFECTS OF NATURAL AND SYNTHETIC IONOPHORES ON THE ACTION POTENTIAL AND ISOMETRIC CONTRACTION OF CARDIAC PURKINJE FIBERS.” Fed. Proceedings, 37.3, 1898, 1978.
4. Gelles JM, Zeiler RH, and Krasnow N: “ELECTRO-MECHANICAL COUPLING IN CARDIAC PURKINJE FIBERS: EFFECTS OF IONOPHORE X-537A.” Bull. N.Y. Aced. Med. 54-3: 316-317, 1978.
5. Gelles JM and Zeiler RH: “ELECTROGENIC HYPERPOLARIZATION IN CANINE CARDIAC PURKINJE FIBERS EXPOSED TO CALCIUM IONOPHORES.” Experientia 34:619, 1978.
6. El-Sherif N, Gomes JAC, Kelen GJ, Khan RG, Kang PS, and Zeiler RH: “ELECTRO-PHYSIOLOGIC, BIOCHEMICAL AND PHARMACOLOGICAL ASPECTS OF REENTRANT VENTRICULAR ARRHYTHMIAS IN THE LATE MYOCARDIAL INFARCTION PERIOD. “IN: “SUDDEN DEATH.”
7. El-Sherif N, Gomes JAC, Kelen GJ, Khan RG Kang PS, and Zeiler RH: “ELECTRO-PHYSIOLOGY OF REENTRANT VENTRICULAR ARRHYTHMIAS IN THE LATE MYOCARDIAL INFARCTION PERIOD.” In: “New Trends in Medical and Surgical Management of Tachyarrhythmias.” Editors, L. Seipl, H.D. Schulti. 1980.
8. El-Sherif N, Zeiler RH, Gough W: “EFFECTS OF CATECHOLAMINES, VERAPA¬MIL AND TETRODOTOXIN ON TRIGGERED AUTOMATICITY IN CANINE ISCHEMIC PURKINJE FIBERS.” Circulation 62: Suppl. III; 1076, 1980.
9. Zeiler RH, Gough WB, Sung R, El-Sherif N: “ELECTROPHYSIOLOGIC EFFECT OF PROPAFENONE IN CANINE ISCHEMIC CARDIAC CELLS.” Am. J. Cardiol, 47:483, 1981.
10. El-Sherif N, Gough, WB, Zeiler RH, Mehra R: “EPICARDIAL MAPPING OF TRIGGERED
AUTOMATICITY IN CANINE ISCHEMIC PURKINJE FIBERS.” Am. J. Cardiol., 47:489, 1981.
11. Gough WB, Zeiler RH, El-Sherif N: “THE ANTIARRHYTHMIC ACTION OF NIFEDIPINE ON TRIGGERED ACTIVITY IN ONE DAY OLD ISCHEMIC ENDOCARDIUM.” Circulation 64: Suppl. IV; 274, 1981.
12. El-Sherif N, Gough WB, Zeiler RH, Mehra R: “DIFFERENT MECHANISMS FOR SPONTANEOUS AND INDUCED VENTRICULAR RHYTHMS IN 24 HOUR-OLD MYOCARDIAL INFARCTION IN THE DOG.” Circulation 64: Suppl. IV; 218, 1981.
13. Mehra R, Zeiler RH, Gough WB, El-Sherif N: “MECHANISM OF REPETITIVE VENTRICULAR RESPONSES RVR’S BLOCK.” Circulation 64: Suppl. IV; 172, 1981.
14. Gough WB, Zeiler RH, Barreca P, El-Sherif N: “THE HYPOTENSIVE EFFECTS OF COMMERCIAL INTRAVENOUS AMIODARONE IN DOGS: DEPENDENCE ON THE DILUENT POLYSORBATE 80.” J. of Cardiovascular Pharm., 4:375-380, 1982.
15. Mehra R, Kelen GJ, Zeiler RH, Zephiran D, Fried P, Gomes JA, El-Sherif N: “NON-INVASIVE HIS BUNDLE ELECTROGRAM: VALUE OF THREE VECTOR LEAD RECORDINGS.” Am. J. Cardiol., 49:344-348, 1982.
16. El-Sherif N, Mehra R, Gough WB, Zeiler RH: “VENTRICULAR RHYTHMS IN CANINE ONE-DAY-OLD MYOCARDIAL INFARCTION. EVIDENCE FOR FOCAL AND REENTRANT MECHANISMS.” Circulation Research, 51:152-166, 1982.
17. Mehra R, Zeiler RH, Gough WB, El-Sherif N: “REENTRANT VENTRICULAR ARRHYTHMIAS IN THE LATE MYOCARDIAL INFARCTION PERIOD. 9. ELECTROPHYSIOLOGICAL ANATOMICAL CORRELATION OF REENTRANT CIRCUITS.” Circulation. 67(1):11-24, January, 1983.
18. Gough WB, Zeiler RH, El-Sherif N: “EFFECTS OF CALCIUM AND CALCIUM ANTAGONISTS ON TRIGGERED ACTIVITY IN ONE-DAY-OLD CANINE ISCHEMIC ENDOCARDIUM.” Am J. Cardiol., 49:914, 1982.
19. El-Sherif N, Mehra R, Gough WB, Zeiler RH: “MECHANISM OF REENTRANT RHYTHMS INDUCED BY BURSTS OF RAPID VENTRICULAR STIMULATION IN THE ISCHEMIC CANINE HEART.” Am J. Cardiol., 49:934, 1982.
20. Zeiler RH, Strand FL, El-Sherif N: “CANINE LEFT ATRIAL TISSUE SPECIFICALLY BINDS ADRENOCORTICOTROPIC HORMONE.” Am. J. Cardiol., 49:1037, 1982.
21. Zeiler RH, Strand FL, El-Sherif N: “ELECTROPHYSIOLOGICAL AND CONTRAC¬TILE RESPONSES OF CANINE ATRIAL TISSUE TO ADRENOCORTI¬COTRO¬PIN.” Peptides, 3:815, 1982.
22. Zeiler RH, Gough WB, El-Sherif N: “ROLE OF CA2+ AND NA2+ ON AFTER DEPOLARIZATIONS IN CANINE ISCHEMIC PURKINJE FIBERS.” Circulation, 66:II-78, 1982.
23. Gough WB, Zeiler RH, El-Sherif N: “BASIS FOR REDUCED TRANSMEMBRANE POTENTIALS ASSORTED WITH TRIGGERED ACTIVITY IN ISCH¬EMIC SUBENDOCARDIAL PURKINJE FIBERS.” Circulation, 66:II-156, 1982.
24. Hariman RJ, Zeiler RH, Gough WB, El-Sherif N: “THE EFFECT OF OUABAIN ON TRIGGERED ACTIVITY IN ONE DAY ONE CANINE INFARCTION.: Circulation, 66:II, 1982.
25. El-Sherif N, Gough WB, Zeiler RH, Mehra R: “VENTRICULAR RHYTHMS IN ONE-DAY-OLD CANINE INFARCTION ARE DUE TO TRIGGERED ACTIVITY.” Circulation, 66:II-357, 1982.
26. El-Sherif N, Mehra R, Gough WB, Zeiler RH: “TERMINATION OF REENTRANT CIRCUITS IN CANINE INFARCTION BY CRYOTHERMAL TECH¬NIQUES.” Circulation, 66:II-358, 1982.
27. El-Sherif N, Mehra R, Gough WB, Zeiler RH: “VENTRICULAR ACTIVATION PATTERNS OF SPONTANEOUS AND INDUCED VENTRICULAR RHYTHMS IN CANINE ONE-DAY-OLD MYOCARDIAL INFARCTION.” Circulation Research, 51:152-166, 1982.
28. El-Sherif N, Mehra R, Gough WB, Zeiler RH: “VENTRICULAR ACTIVATION PATTERNS OF PLEOMORPHIC VENTRICULAR TACHYCARDIAS THAT TERMINATE SPONTANEOUSLY OR DEGENERATE INTO VENTRICULAR FIBRILLATION.” J. American College of Cardiology, 2:621, 1983.
29. Gough WB, Zeiler RH, El-Sherif N: “EFFECTS OF DILTIAZEM ON TRIGGERED ACTIVITY IN ONE-DAY-OLD ISCHEMIC ENDOCARDIUM OF THE DOG.” J. American College of Cardiology, 2:692, 1983.
30. Mehra E, Zeiler RH, Gough WB, El-Sherif N: “REENTRANT VENTRICULAR ARRHYTHMIAS CORRELATION OF REENTRANT CIRCUITS. THE ANATOM¬ICAL BASIS FOR REENTRY.” Circulation 67(1):11-24, 1983.
31. El-Sherif N, Mehra R, Gough WB, Zeiler RH: “EFFECTS OF REVERSIBLE COOLING ON REENTRANT TACHYCARDIA IN CANINE INFARCTION.” Pace, 6:5, 1983.
32. El-Sherif N, Gough WB, Zeiler RH, Mehra R: “TRIGGERED RHYTHMS IN ONE-DAY-OLD MYOCARDIAL INFARCTION IN THE DOG.” Circulation Research, 52:566-579, 1983.
33. El-Sherif N, Mehra R, Gough WB, Zeiler RH: “REENTRANT VENTRICULAR ARRHYTHMIAS IN THE LATE MYOCARDIAL INFARCTION PERIOD AND INTERRUPTION OF REENTRANT CIRCUITS BY CRYOTHERMAL TECHNIQUES.” Circulation, 63:644-656, 1983.
34. El-Sherif N, Mehra R, Gough WB, Zeiler RH: “EFFECTS OF REVERSIBLE COOLING ON REENTRANT TACHYCARDIAS IN CANINE INFARCTION.” Physiologist 26:A91, 1983.
35. Gough WB, Zeiler RH, El-Sherif N: “EFFECTS OF CAFFEINE ON TRIGGERED ACTIVITY IN ONE-DAY-OLD ISCHEMIC ENDOCARDIUM OF THE DOG.” Circulation 68:III-20, 1983.
36. El-Sherif N, Mehra R, Gough WB, Zeiler RH: “BURST PACING VERSUS PREMATURE STIMULATION IN THE INDUCTION OF REENTRY.” J. Amer. Coll. Cardiol. 3:587, 1984.
37. Mehra R, Gough WB, Zeiler RH, El-Sherif N: “MECHANISM OF LIDOCAINE ACTION ON REENTRANT VENTRICULAR RHYTHMS IN THE CANINE ISCHEMIC HEART.” J. Amer. Coll. Cardiol. 3:542, 1984.
38. Gough WB, Zeiler RH, El-Sherif N: “EFFECTS OF NIFEDIPINE ON TRIGGERED ACTIVITY IN ONE-DAY-OLD MYOCARDIAL INFARCTION IN DOGS.” Amer. J. Cardiol. 53:303-306, 1984.
39. Mehra R, Gough WB, Zeiler RH, El-Sherif N: “DUAL VENTRICULAR STIMULA¬TION FOR PREVENTION OF REENTRANT VENTRICULAR ARRHYTH¬MIAS.” J. Amer. Coll. Cardiol. 3:472, 1984.
40. Gough WB, Zeiler RH, El-Sherif N: “DEPENDENCE OF TRIGGERED ACTIVITY ON DIASTOLIC POTENTIALS IN ONE-DAY-OLD ISCHEMIC PURKINJE FIBERS.” J. Amer. Coll. Cardiol. 3:477, 1984.
41. El-Sherif N, Gough WB, Zeiler RH: “THE EFFECT OF DIFFERENTIAL SHORTENING OF REFRACTORINESS IN SUCCESSIVE SHORT CARDIAC CYCLES ON THE INITIATION AND TERMINATION OF REENTRY IN THE ISCHEMIC CANINE HEART.” J. Amer. Coll. Cardiol. 3:477, 1984.
42. Zeiler RH, Gough WB, El-Sherif N: “ELECTROPHYSIOLOGIC EFFECT OF PROPAFENONE ON CANINE ISCHEMIC CARDIAC CELLS.” Amer. J. Cardiol. 54:424-429, 1984.
43. El-Sherif N, Mehra R, Gough WB, Zeiler RH: “REENTRANT VENTRICULAR ARRHYTHMIAS IN THE LATE MYOCARDIAL INFARCTION PERIOD: II. BURST PACING VERSUS MULTIPLE PREMATURE STIMULATION IN THE INDUCTION OF REENTRY.” J. Amer. Coll. Cardiol. 4:295-304, 1984.
44. Zeiler RH, Tobiasz C, Henkin R, Gough WB, El-Sherif N: “THE EFFECTS OF ISCHEMIA ON INTRACELLULAR POTASSIUM ACTIVITY AND MEMBRANE POTENTIAL IN CANINE ENDOCARDIAL TISSUE.” Circulation, 70(4): 898, 1984.
45. Gough WB, Zeiler RH, El-Sherif N: “EFFECTS OF DILTIAZEM ON TRIGGERED ACTIVITY IN CANINE ONE-DAY-OLD INFARCTION. Cardiovasc Res 18:339-343, 1984.
46. El-Sherif N, Gough WB, Hariman R, Zeiler RH: “MECHANISMS OF TERMINATION OF ACCELERATION OF REENTRANT TACHYCARDIA BY BURST PACING.” Circulation, 70:II-91, 1984.
47. Hariman RH, Zeiler RH, Gough WB, El-Sherif N: “ENHANCEMENT OF TRIGGERED ACTIVITY IN ISCHEMIC PURKINJE FIBERS BY OUABAIN. A MECHANISM OF INCREASED SUSCEPTIBILITY TO DIGITALIS TOXICITY IN MYOCARDIAL INFARCTION.” J. Amer. Coll. Card. 5(3):672-679, 1985.
48. El-Sherif N, Gough WB, Hariman R, Zeiler RH: “ROLE OF NON UNIFORM REFRACTORY DISTRIBUTION VERSUS ANISOTROPIC ANATOMIC PROPERTIES IN THE INITIATION OF REENTRANT EXCITATION IN THE CANINE POST-INFARCTION HEART.” J. Amer. Coll. Card. 5:390, 1985.
49. El-Sherif N, Gough WB, Zeiler RH, and Hariman R: “REENTRANT VENTRICULAR ARRHYTHMIAS IN THE LATE MYOCARDIAL INFARCTION PERIOD. 12. SPONTANEOUS VERSUS INDUCED REENTRY AND INTRA¬MURAL VERSUS EPICARDIAL CIRCUITS. J. Am. Coll. Cardiol. 6(1):124-132, 1985.
50. Gough WB, Mehra R, Restive M, Zeiler RH and El-Sherif N: “REENTRANT VENTRICULAR ARRHYTHMIAS IN THE LATE MYOCARDIAL INFARCTION PERIOD IN DOG. 13. CORRELATION OF ACTIVATION AND REFRACTORY MAPS.” Circ. Res. 57(3):432-442, 1985.
51. El-Sherif N, Gough WB, Zeiler RH, Craelius W, and Restivo M: “ROLE OF NON UNIFORM REFRACTORY DISTRIBUTION VERSUS ANISOTROPIC PROPERTIES IN THE INITIATION OF REENTRANT EXCITATION IN THE CANINE POST INFARCTION HEART.” J. Amer. Coll. Cardiol. 5(2):390, 1985.
52. Fontaine JM, Zeiler RH, Henkin R, El-Sherif N: “SIMULTANEOUS MONOPHASIC ACTION POTENTIAL RECORDING AND REGIONAL ENDOCARDIAL REFRACTORY PERIOD DETERMINATION USING A NEW DUAL PUR¬POSE CONTACT ELECTRODE CATHETER., PACE 9(2):278, 1986.
53. Craelius W, Chen V, El-Sherif N, and Zeiler RH: “IN VIVO RECORDING OF EARLY AFTER DEPOLARIZATION PRECEDING TORSADES DE POINTES.” J. Amer. Coll. Cardiol. 7:124A, 1986.
54. Kelen GJ, Henkin R, Restivo M, Zeiler RH, Caref EB, and El-Sherif N: “SIGNAL AVERAGING OF HIGH GAIN HOLTER ECG RECORDINGS VALIDATION OF A NEW TECHNIQUE FOR DETECTION OF AFTER POTENTIALS.” J. Amer. Coll. Cardiol. 7:104A, 1986.
55. Zeiler RH, Sequeira JM, Henkin R, Sedlis SP, El-Sherif N: “LYSOPHOSPHATIDYL CHOLINE: PROBABLE AGENT FOR MAINTAINED TRIGGERED ACTIVITY IN ISCHEMIC CARDIAC PURKINJE FIBERS.” J. Amer. Coll. Cardiol. 9:252, 1987.
56. El-Sherif N, Zeiler RH, Craelius W, Henkin R, Gough WB: “BRADYCARDIA-DEPENDENT QTU PROLONGATION AND TORSADES-DE-POINTES DUE TO EARLY AFTER-DEPOLARIZATIONS.” Circulation 76:429, 1987.
57. El-Sherif N, Zeiler RH, Craelius W, Gough WB, Henkin R: “2:1 BLOCK OF AN EARLY AFTER-DEPOLARIZATION AS A MECHANISM FOR TB ALTERNANS.” J. Amer. Coll. Cardiol. 11:254A, 1988.
58. El-Sherif N, Zeiler RH, Craelius W, Gough WB, Henkin R. QTU PROLONGATION AND POLYMORPHIC VENTRICULAR TACHYARRHYTHMIAS DUE TO BRADYCARDIA DEPENDENT EARLY AFTERDEPOLARIZATIONS. Circ Res. 1988; 63:286-305.
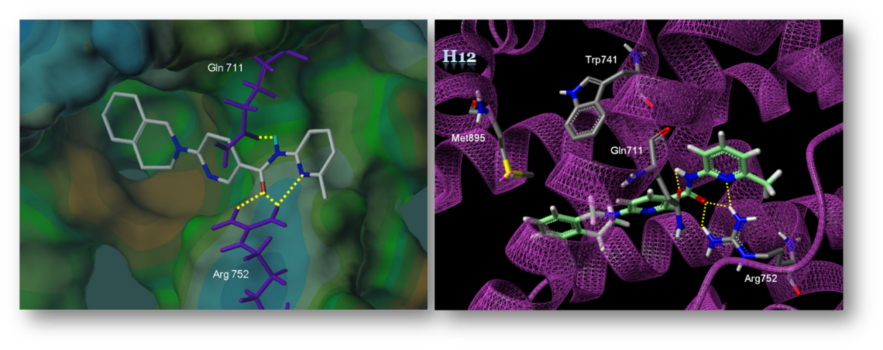 Prostate cancer is the most commonly diagnosed malignancy and the second leading cause of cancer deaths in men in the United States. Antiandrogen therapy with first-generation androgen receptor (AR) antagonists, such as bicalutamide (BIC), leads to a temporary reduction of prostate cancer with a decrease in the level of serum prostate-specific antigen (PSA), a biomarker of prostate cancer. Unfortunately, cancer cells grow again in the absence of androgens and progress to castration-resistant prostate cancer (CRPC). CRPC is attributed to elevated AR gene expression which can be driven by AR gene amplification, AR gene mutation, or ligand-independent AR activation. In addition, the first-generation AR antagonists acquire agonistic property in cells that are engineered to express higher amounts of AR. This switch from antagonism to partial agonism is demonstrated by the anti-androgen withdrawal syndrome; the serum concentration of PSA decreases in patients after discontinuation of anti-androgens.
Prostate cancer is the most commonly diagnosed malignancy and the second leading cause of cancer deaths in men in the United States. Antiandrogen therapy with first-generation androgen receptor (AR) antagonists, such as bicalutamide (BIC), leads to a temporary reduction of prostate cancer with a decrease in the level of serum prostate-specific antigen (PSA), a biomarker of prostate cancer. Unfortunately, cancer cells grow again in the absence of androgens and progress to castration-resistant prostate cancer (CRPC). CRPC is attributed to elevated AR gene expression which can be driven by AR gene amplification, AR gene mutation, or ligand-independent AR activation. In addition, the first-generation AR antagonists acquire agonistic property in cells that are engineered to express higher amounts of AR. This switch from antagonism to partial agonism is demonstrated by the anti-androgen withdrawal syndrome; the serum concentration of PSA decreases in patients after discontinuation of anti-androgens.